Itch Response Data
VIEW THE RESULTS
Approved for patients ages 12 years and older. Click here for different pediatric patient dosing.

Kalvin, a real Adbry patient. Individual results may vary.
The efficacy and safety of Adbry were assessed in 3 randomized, double-blind, placebo-controlled trials1

Q2W=every 2 weeks; Q4W=every 4 weeks; TCI=topical calcineurin inhibitor; TCS=topical corticosteroid.
Adbry was administered via subcutaneous injection.
*Responders defined as achieving the primary endpoints of IGA 0 or 1 or EASI-75 at Week 16.
Nonresponders at Week 16 and subjects who lost clinical response during the maintenance period were placed on open-label treatment with Adbry 300 mg every other week and optional use of TCS.1
†Placebo responders continued on placebo to maintain blind participation and were not included in analyses after Week 16.1,2
Adbry was administered via subcutaneous injection.
*Responders defined as achieving the primary endpoints of IGA 0 or 1 or EASI-75 at Week 16. Subjects who did not achieve clinical response at Week 16 received Adbry 300 mg every other week+TCS for another 16 weeks.1,3
†A midpotency TCS (ie, mometasone furoate 0.1% cream) was dispensed at each dosing visit. The subjects were instructed to apply a thin film of the dispensed TCS as needed, once daily, to active lesions from Week 0 to Week 32, and were to discontinue treatment with TCS when control was achieved. An additional, lower-potency TCS or TCI could be used at the investigator’s discretion on areas of the body where use of the supplied TCS was not advisable, such as areas of thin skin.
‡Placebo responders continued on placebo to maintain blind participation and were not included in analyses after Week 16.1,2
Improvement in atopic dermatitis lesions at Week 16

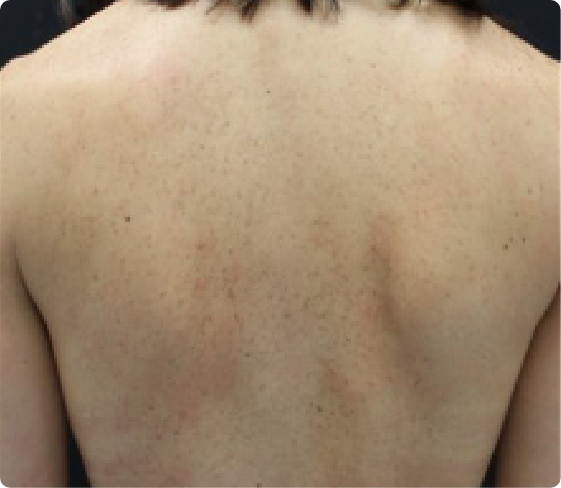
BEFORE TREATMENT
EASI = 31.9
IGA = 4
Worst Daily Pruritus NRS = 8
AT WEEK 16
EASI = 3.6
IGA = 2
Worst Daily Pruritus NRS = 3
Example of improvement in EASI from baseline to Week 16 in a patient receiving Adbry in the ECZTRA 2 study.3 Individual patient results may vary.
Limitations:
Analyses of TCS use as needed were based on the provided TCS only (mometasone furoate 0.1% cream provided every other week applied once daily as needed). Additional low potency TCS and TCI were allowed as needed and not accounted for in this analysis. Analyses were prespecified but not adjusted for multiplicity. Conclusions should be made with caution.
Q2W=every 2 weeks; Q4W=every 4 weeks; TCS=topical corticosteroid.
*Responders were defined as subjects with an IGA 0 or 1 (“clear” or “almost clear”) or EASI-75 at Week 16. At Week 16, responders were re-randomized to Adbry 300 mg Q2W, Adbry Q4W, or placebo every other week for another 36 weeks.
†Subjects who received rescue treatment or with missing data were considered nonresponders.
The ECZTEND OLE trial is an ongoing long-term assessment of safety and efficacy4
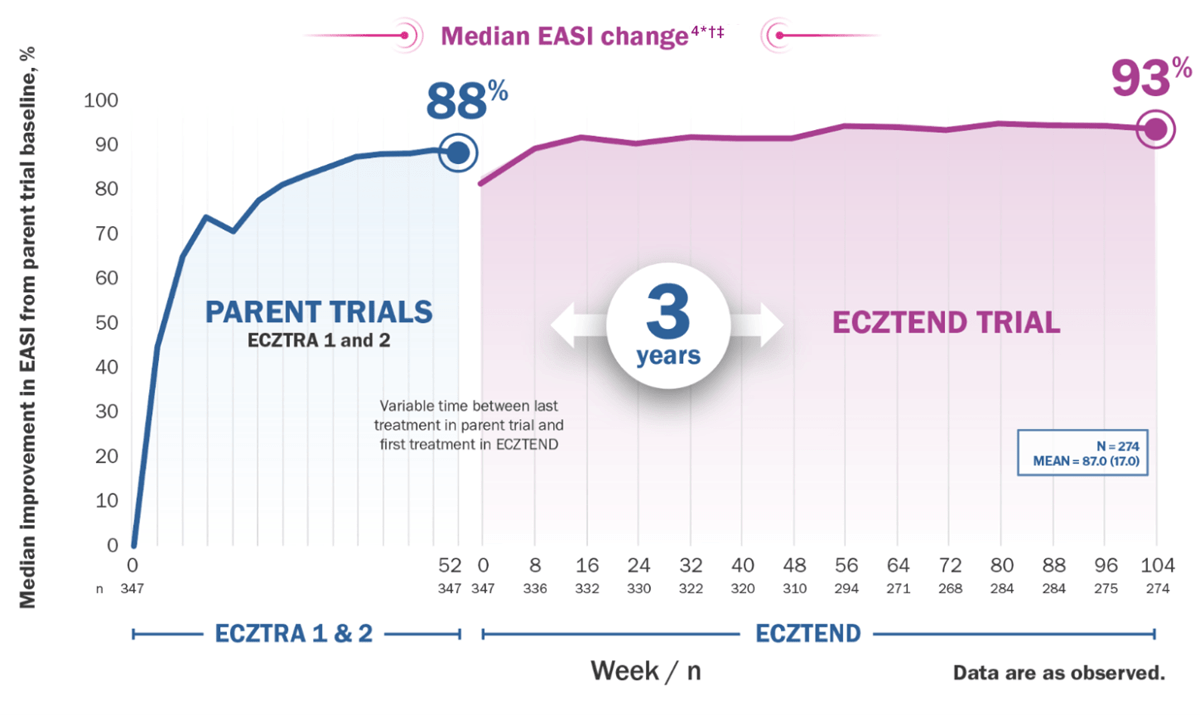
*Median EASI score at parent trial baseline: 26.7; at ECZTEND baseline: 4.7; at Week 104: 2.0.
†Variable time between last treatment in parent trial and first treatment in ECZTEND (maximum 26 weeks).
‡A total of 1430 patients were enrolled in ECZTEND at data cutoff. Data presented from a post-hoc interim analysis of an ongoing OLE trial represents a selected subgroup of patients (n=274) who were eligible, chose to enter from 2 of the parent trials (ECZTRA 1 and ECZTRA 2), and were treated for 3 years by the time of data cutoff (April 30, 2021), and as such, the data may not be generalizable to the full Adbry population.
Limitations:
Limitations and context associated with the open-label study design and data are described above and include decreasing sample size, potential continued involvement of responders, and attrition of nonresponders. Data presented are descriptive in nature and no statistical comparisons are made
*Most commonly reported as common cold.
ADBRY® (tralokinumab-ldrm) injection is indicated for the treatment of moderate-to-severe atopic dermatitis in patients aged 12 years and older whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. ADBRY can be used with or without topical corticosteroids.
Please see full Prescribing Information.
1. Adbry Prescribing Information, LEO Pharma. 2. Wollenberg A, Blauvelt A, Guttman-Yassky E, et al; ECZTRA 1 and ECZTRA 2 study investigators. Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br J Dermatol. 2021;184(3):437-449. 3. Data on file. LEO Pharma. 4. Langley R, Reich K, Simpson E, et al. Long-term improvements in disease severity, itch, and quality of life after 3 years of tralokinumab treatment in adults with moderate-to-severe atopic dermatitis. Poster presented at 4th Annual Revolutionizing Atopic Dermatitis Conference, April 9-11, 2022. 5. Bieber T. Interleukin-13: targeting an underestimated cytokine in atopic dermatitis. Allergy. 2020;75(1): 54-62. 6. Tsoi LC, Rodriguez E, Degenhardt F, et al. Atopic dermatitis is an IL-13-dominant disease with greater molecular heterogeneity compared to psoriasis. J Invest Dermatol. 2019;139(7):1480-1489. 7. Kim BE, Leung DYM, Boguniewicz M, Howell MD. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin Immunol. 2008;126(3):332-337. 8. Szegedi K, Lutter R, Res PC, et al. Cytokine profiles in interstitial fluid from chronic atopic dermatitis skin. J Eur Acad Dermatol Venereol. 2015 Nov;29(11):2136-44. 9. Silverberg JI, Kantor R. The role of interleukins 4 and/or 13 in the pathophysiology and treatment of atopic dermatitis. Dermatol Clin. 2017;35(3):327-334. 10. Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD. Atopic dermatitis. Nat Rev Dis Primers. 2018;4(1):1. 11. Popovic B, Breed J, Rees DG, et al. Structural characterisation reveals mechanism of IL-13-neutralising monoclonal antibody tralokinumab as inhibition of biding to IL-13Rα1 and IL-13Rα2. J Mol Biol. 2017;429(2):208-219. 12. Silverberg JI, Toth D, Bieber T, et al; ECZTRA 3 study investigators. Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: results from the double-blind, randomized, multicentre, placebo-controlled phase III ECZTRA 3 trial. Br J Dermatol. 2021;184(3):450-463. 13. Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol. 2001;10(1):11-18. 14. Leshem YA, Hajar T, Hanifin J, Simpson E. What the Eczema Area and Severity Index score tells us about the severity of atopic dermatitis: an interpretability study. Br J Dermatol. 2015;172(5):1353-1357. 15. Chopra R, Vakharia PP, Sacotte R, et al. Severity strata for Eczema Area and Severity Index (EASI), modified EASI, Scoring Atopic Dermatitis (SCORAD), objective SCORAD, Atopic Dermatitis Severity Index and body surface area in adolescents and adults with atopic dermatitis. Br J Dermatol. 2017;177(5):1316-1321. 16. Futamura M, Leshem YA, Thomas KS, et al. A systematic review of Investigator Global Assessment (IGA) in atopic dermatitis (AD) trials: many options, no standards. J Am Acad Dermatol. 2016;74(2):288-294. 17. Phan NQ, Blome C, Fritz F, et al. Assessment of pruritus intensity: prospective study on validity and reliability of the visual analogue scale, numerical rating scale and verbal rating scale in 471 patients with chronic pruritus. Acta Derm Venereol. 2012;92(5):449-581.