
One-time initial loading dose3:
2 x 300-mg autoinjectors (600 mg)
Strongly recommended by AAD and AAAAI guidelines as a systemic therapy for moderate-to-severe atopic dermatitis in adult patients1,2
ADULT
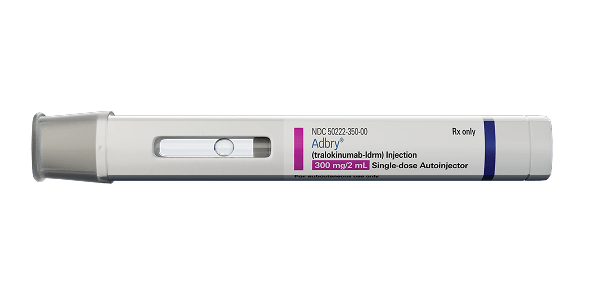
Not actual size.
*q4w for adult patients <220 lbs who achieve clear or almost clear skin after 16 weeks.
Actor portrayal.

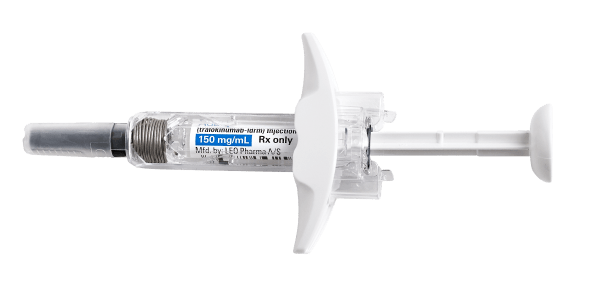
Not actual size.
*Option is available for adults <100 kg (220 lbs) who achieve clear or almost clear skin after 16 weeks on Adbry.
Not an actual patient. Individual results may vary.
ADBRY® (tralokinumab-Idrm) injection is indicated for the treatment of moderate-to-severe atopic dermatitis in patients aged 12 years and older whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable. ADBRY can be used with or without topical corticosteroids.
Please see full Prescribing Information.
1. Davis DMR, et al. J Am Acad Dermatol. 2024:90(2):e43-e56. doi:10.1016/j.jaad.2023.08.102. 2. AAAAI/ACAAI JTF Atopic Dermatitis Guideline Panel, et al. Ann Allergy Asthma Immunol. 2024;132(3):274-312. doi:10.1016/j.anai.2023.11.009. 3. ADBRY. Prescribing information. LEO Pharma Inc.